
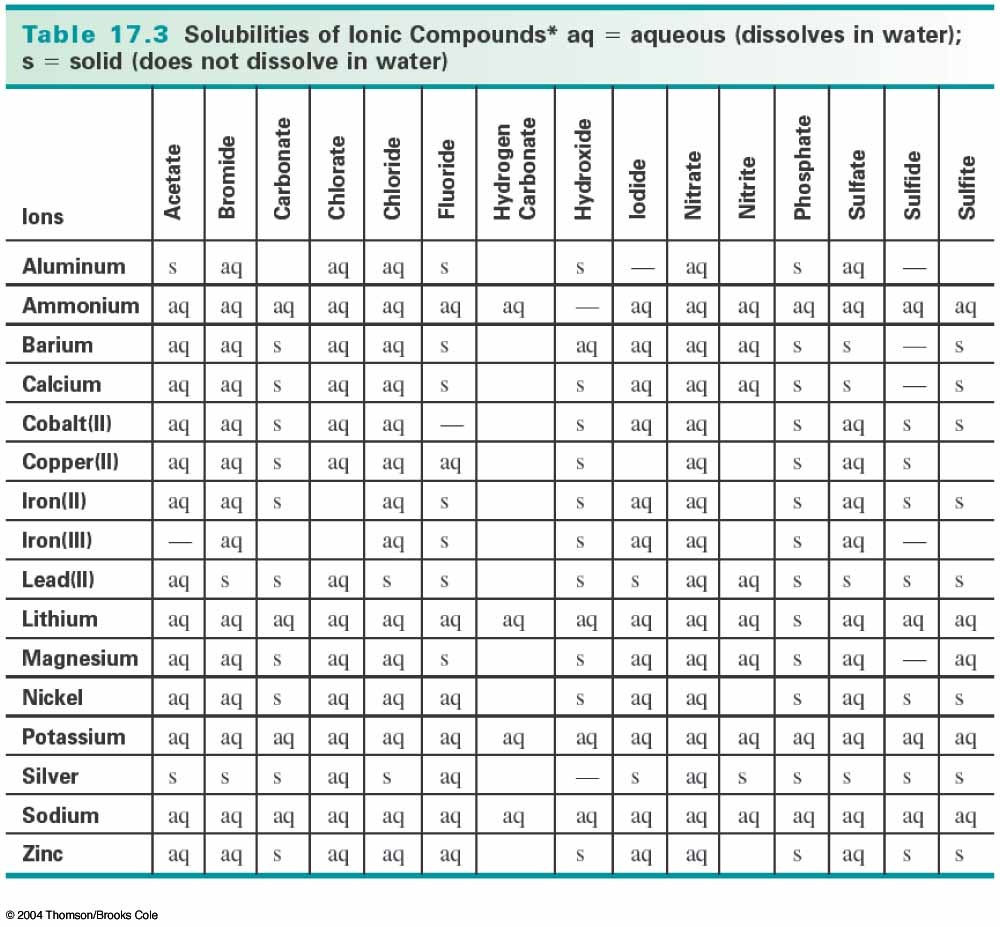
The products should rearrange the ions to: What would be the expected products and will a precipitate form? The resulting balanced reaction would be:Ģ AgNO 3(aq) + MgBr 2 → 2 AgBr(s) + Mg(NO 3) 2(aq) The other compound Mg(NO 3) 2 will remain in solution because all nitrates, (NO 3) -, are soluble in water. Are the products soluble in water?Īccording to the solubility rules, all silver salts are insoluble in water with the exception of silver nitrate, silver acetate and silver sulfate. The state of the products needs to be determined. The balanced reaction would be:Ģ AgNO 3(aq) + MgBr 2 → 2 AgBr(?) + Mg(NO 3) 2(?)

For example, a silver nitrate solution (AgNO 3) is mixed with a solution of magnesium bromide (MgBr 2). The question remains, will AD or CB remain in solution or form a solid precipitate?Ī precipitate will form if the resulting compound is insoluble in water. This reaction is generally a double replacement reaction in the form: When two aqueous solutions are mixed, the ions interact to form products. These solutions are represented in chemical equations in the form: AB(aq) where A is the cation and B is the anion.
WHICH COMBINATION WILL PRODUCE A PRECIPITATE HOW TO
This guide will show how to use the solubility rules for inorganic compounds to predict whether or not the product will remain in solution or form a precipitate.Īqueous solutions of ionic compounds are comprised of the ions making up the compound dissociated in water. Next, learn about the Effects of Acid Rain.When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. The Long-Term Monitoring (LTM) Network measures and monitors surface water chemistry at over 280 sites to provide valuable information on aquatic ecosystem health and how water bodies respond to changes in acid-causing emissions and acid deposition. When acid deposition is washed into lakes and streams, it can cause some to turn acidic. Air concentrations are measured by CASTNET at more than 90 locations. Dry deposition estimates for nitrogen and sulfur pollutants are provided by the Clean Air Status and Trends Network (CASTNET). Unlike wet deposition, dry deposition is difficult and expensive to measure. The NADP/NTN collects acid rain at more than 250 monitoring sites throughout the US, Canada, Alaska, Hawaii and the US Virgin Islands. Policymakers, research scientists, ecologists, and modelers rely on the National Atmospheric Deposition Program’s (NADP) National Trends Network (NTN) for measurements of wet deposition. Acid rain usually has a pH between 4.2 and 4.4. Normal rain has a pH of about 5.6 it is slightly acidic because carbon dioxide (CO 2) dissolves into it forming weak carbonic acid. The lower a substance's pH (less than 7), the more acidic it is the higher a substance's pH (greater than 7), the more alkaline it is. For example, in desert areas the ratio of dry to wet deposition is higher than an area that receives several inches of rain each year.Īcidity and alkalinity are measured using a pH scale for which 7.0 is neutral.

The amount of acidity in the atmosphere that deposits to earth through dry deposition depends on the amount of rainfall an area receives. When the accumulated acids are washed off a surface by the next rain, this acidic water flows over and through the ground, and can harm plants and wildlife, such as insects and fish. The acidic particles and gases may deposit to surfaces (water bodies, vegetation, buildings) quickly or may react during atmospheric transport to form larger particles that can be harmful to human health. Dry DepositionĪcidic particles and gases can also deposit from the atmosphere in the absence of moisture as dry deposition. The sulfuric and nitric acids formed in the atmosphere fall to the ground mixed with rain, snow, fog, or hail. Wet deposition is what we most commonly think of as acid rain. Winds can blow SO 2 and NO X over long distances and across borders making acid rain a problem for everyone and not just those who live close to these sources. Manufacturing, oil refineries and other industries.Two thirds of SO 2 and one fourth of NO X in the atmosphere come from electric power generators. Burning of fossil fuels to generate electricity.The major sources of SO 2 and NO X in the atmosphere are:
While a small portion of the SO 2 and NO X that cause acid rain is from natural sources such as volcanoes, most of it comes from the burning of fossil fuels. These then mix with water and other materials before falling to the ground. The SO 2 and NO X react with water, oxygen and other chemicals to form sulfuric and nitric acids. Acid rain results when sulfur dioxide (SO 2) and nitrogen oxides (NO X) are emitted into the atmosphere and transported by wind and air currents.


 0 kommentar(er)
0 kommentar(er)
